Due to increasing COVID-19 case counts across many states, public health officials are recommending source control measures to reduce disease transmission, including mandatory mask use in public places. Unfortunately, these measures rely upon the user’s effective compliance, both in work and in social settings. Growing evidence suggests that aerosols may play a part in the transmission of SARS-CoV-2 and that engineering control measures are needed in public facilities like office buildings and schools to reduce the risk of disease transmission. These measures can be effective for immediate control of aerosol exposure in the indoor environment.
The current control recommendations are based on the belief that SARS-CoV-2 is transmitted by large exhaled droplets. The Centers for Disease Control and Prevention (CDC) recommends using protective measures to guard against this mode of transmission, including wearing masks or face coverings, social distancing, hand-washing, and surface cleaning and disinfection. The CDC identifies close person-to-person contact (within 6 feet) as the most important mode of transmission for SARS-CoV-2 because there is risk of directly contacting respiratory droplets expelled from an infected individual when he or she sneezes, coughs, or talks.[1] The CDC also recognizes the potential to contract COVID-19 by touching contaminated surfaces and transferring the virus to the mouth, nose, or eyes but that this indirect contact is not thought to be the main way the virus is spread.
Recent lines of evidence suggest that aerosols may play a part in SARS-CoV-2 (COVID-19) disease transmission. To this point, the CDC states in COVID-19 transmission documentation that “… we are still learning more about how this virus spreads.” 1 While the weight of evidence continues to grow, the World Health Organization (WHO) issued a scientific brief, in part as a response to an open letter signed by 239 scientists from across the globe (Morawska et al., 2020).[2] In the brief, the WHO acknowledges that there is a possibility of aerosol transmission in crowded indoor spaces where ventilation is inadequate. However, it stops short of recognizing the aerosol transmission route, as it finds that “much more research is needed given the possible implications of such route of transmission.”[3] As of July 2020, no governmental or public health agencies have issued guidance for controlling aerosols in non-healthcare settings.
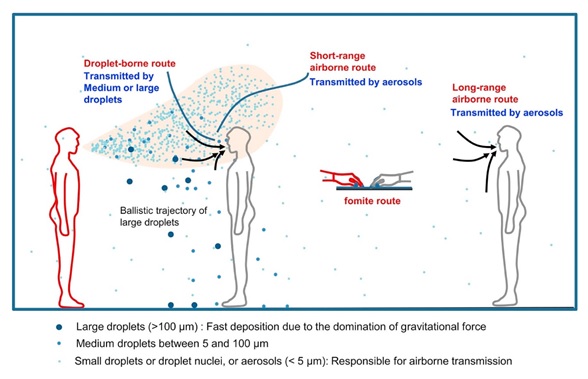
Transmission of an infectious agent from respiratory secretions is typically classified by public health agencies (e.g., the CDC) as respiratory droplets (>5 micrometers (µm) in diameter) or droplet nuclei (≤5 µm in diameter). Regardless of name, both droplet types are classified as aerosols under the traditional industrial hygiene definition (i.e., a collection of solid or liquid particles dispersed in a gas, like air). Aerosols of biological origin (e.g., respiratory droplets and droplet nuclei) are generated from people when they sneeze, cough, or talk and typically range up to 100 µm in diameter. In general, an aerosol’s physical characteristics, including its size, shape, and density, determine how long it remains suspended in the air, as illustrated in the figure below. Larger aerosols will settle more quickly due to gravity and air currents, whereas smaller aerosols will travel farther.
The SARS-CoV-2 dose that results in a case of COVID-19 has not yet been established. While the infectious dose or concentration is currently unknown, lack of comprehensive infectious dose data has not stopped the use of control measures for other infectious agents. For example, the CDC states, “Information on the transmission parameters of M. tuberculosis is also incomplete. Neither the smallest infectious dose of M. tuberculosis nor the highest level of exposure to M. tuberculosis at which transmission will not occur has been defined conclusively.”[4] While the research on infectious dose and controlling exposure to SARS-CoV-2 will continue, there are prudent actions to reduce indoor exposure that can be taken now that have not yet been widely adopted.
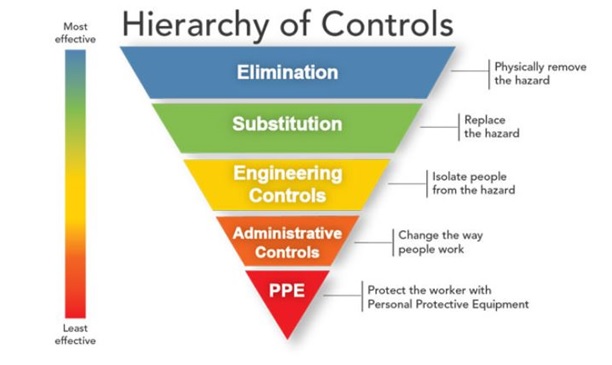
More effective and reliable exposure control measures are needed, as the weight of evidence supports the aerosol transmission of SARS-CoV-2 in the general population. The concept of small-size aerosol generation and transmission is important to understand the risk of spreading disease from infected individuals (including presymptomatic and asymptomatic carriers) to noninfected individuals. Current control methods rely upon administrative controls (e.g., social distancing, wearing a face mask, and cleaning) and personal protective equipment (PPE) (mostly N95 respirators, as cloth or surgical masks do not meet the criteria of PPE). In the hierarchy of controls, PPE is the least effective and the last line of defense for controlling a workplace hazard (see figure below). On the other hand, the use of engineering controls for risk reduction takes the responsibility away from the user for a more uniform application in the indoor environment.
Using increased ventilation or filtration to control exposure to aerosolized infectious agents is not a new concept and can be an effective way for reducing exposures to aerosols generated by infected individuals. Increased ventilation rates and enhanced filtration have been used in health care to reduce exposure to infectious agents. For example, in the Guidelines for Environmental Infection Control in Health-Care Facilities (2003), the CDC recommends the use of minimum air exchanges per hour (ACH) in specific areas of a healthcare facility, including but not limited to airborne infection isolation, isolation anterooms, operating rooms, critical care rooms, and protective environments. The recommended ACH rates across these example locations range from ≥ 6 ACH to ≥ 15 ACH. The guidance also recommends the use of filtration as “the first step in achieving acceptable indoor air quality” for healthcare environments.5 Additionally, the CDC guidelines state use of high efficiency particulate air (HEPA)-filtered air recirculation devices can provide supplemental controls for infectious airborne agents.[5]
Examples to support the aerosol transmission route include but are not limited to:
A National Academy of Science correspondence, prompted by a question from the Executive Office of the President on whether SARS-CoV-2 could be spread by conversation and sneeze-/cough-induced droplets, observed that “while the current SARS-CoV-2 specific research is limited, the results of available studies are consistent with aerosolization of virus from normal breathing.”[1]
Anderson et al. (2020) reviewed data available at the time of publication and observed that the current weight of evidence suggests that aerosol transmission is an important COVID-19 disease pathway. Based on the information presented, the authors conclude there is an immediate need to address SARS-CoV-2 aerosol transmission.[2]
A recent open letter regarding SARS-CoV-2 aerosol transmission from Morawska et al. (2020) signed by 239 scientists appeals to relevant national and international bodies, and to the medical community, for the recognition that COVID-19 has the potential for airborne transmission. The correspondence references studies that, in the opinion of the signatories, demonstrate “beyond any reasonable doubt that viruses are released during exhalation, talking, and coughing in microdroplets small enough to remain aloft in air and pose a risk of exposure at distances beyond 1 to 2 m from an infected individual”.[3] Morawska et al. (2020) observe that the airborne transmission pathway is the only plausible explanation for SARS-CoV-2 “superspreader” scenarios in which crowded conditions were present and ventilation was inadequate. The correspondence states that there is “more than enough supporting evidence” to protect against SARS-CoV-2 airborne transmission despite the lack of universal acceptance of this exposure pathway.
A recently published study by Zhen-Dong Guo et al. (2020) in Emerging Infectious Diseases found that the results of the aerosol evaluation performed within a hospital ward in Wuhan, China, confirm that SARS-CoV-2 aerosol exposure poses risks and that the maximum aerosol transmission distance exceeded 6 feet (~2 meters (m)) and may be as far as 13 feet (4 m).[4]
[1] NAS: “Rapid Expert Consultation on the Possibility of Bioaerosol Spread of SARS-CoV-2 for the COVID-19 Pandemic.” (April 1, 2020) (2020). https://www.nap.edu/read/25769/chapter/1#2
[2] Anderson et al. (2020): “Consideration of the Aerosol Transmission for COVID-19 and Public Health.” Risk Analysis. Vol. 40, No. 5.
[3] Morawska et al. It is Time to Address Airborne Transmission of COVID-19. Clinical Infectious Disease (2020).
[4] Zhen-Dong Guo et al. “Aerosol and Surface Distribution of Severe Acute Respiratory Syndrome Coronavirus 2 in Hospital Wards, Wuhan, China, 2020.” Emerging Infectious Diseases. Vol. 26, No. 7 (July, 2020).
Engineering solutions to address SARS-CoV-2 can be effective regardless of the building type and can be implemented into existing mechanical operations. These practical and immediate engineering solutions should be used in existing facilities (e.g., stores, office spaces, schools, etc.) to address the potential aerosol exposure pathway. As Morawska et al. (2020) concluded, exposure pathways important to COVID-19 must be addressed to slow the spread of the disease and to mitigate the risk of airborne transmission.
Building owners or operators can use enhanced filtration in existing heating, ventilation, and air-conditioning (HVAC) systems to lower exposure to SARS-CoV-2-containing aerosols. HEPA filters can remove at least 99.7% of airborne particles 0.3 µm in size. These pleated filters can capture aerosols that are present in indoor ambient air, including human-generated aerosols. Another option is to use enhanced filters with at least a minimum efficiency reporting value (MERV) 13 rating, which filter out at least 50% of the particles in the 0.1 µm–1.0 µm range.[6] If a higher-value MERV filter is used, air flows may need to be adjusted to meet the filter’s design specifications. The manufacturing specification of the HVAC unit should be consulted to ensure that adequate airflow can be achieved.
Another direct and immediate option is to increase the number of air exchanges in a room per hour (ACH) using existing HVAC systems. Six ACH should be the minimum target to control exposure—similar to the recommendation for existing healthcare facilities. A higher density of occupants may necessitate additional ACH. It is important to check the operational limits of existing HVAC units, as the system’s design may not safely allow for increased air flow. Consult with a professional before increasing air flow to ensure the HVAC system can handle the additional workload.
In situations when HVAC system design limits the ability to increase ventilation rates, the use of “off the shelf” portable HEPA air-cleaning systems to recirculate the air within a single space can provide equivalent room air exchanges. These HEPA units are commonly referred to as “air scrubbers” or negative air machines and are designed to enhance air filtration by recirculating a high volume of air through a HEPA filter to capture aerosols from the indoor environment. Use of either ceiling-mounted or portable HEPA machines can effectively reduce infectious aerosol concentrations in a single space,[7] like a classroom, a lobby, or an office.
Use of stand-alone HEPA filtration systems will depend on the air volume of the room, the configuration of the space (including furniture), the number of occupants, the placement of the unit(s) with respect to any existing mechanical ventilation (e.g., HVAC) system installed in the space, and the volume of air that the unit can recirculate per unit time (i.e., cubic feet per minute (CFM)). Professionals should be consulted to ensure the portable unit is not short-circuiting air flow, resulting in air stagnation. Routine maintenance of these units will be required, including filter replacement and unit decontamination. Respiratory protection and other PPE should be worn during decontamination activities to prevent contact with potentially high concentrations of aerosolized virus material.
Another recommended and viable option is to open outside air dampers on fixed HVAC systems to increase the amount of outdoor air introduced into the system. However, this recommendation may have limited application opportunities due to high humidity and the quality of the outdoor air. Introducing outdoor air in high-humidity environments can create favorable conditions for mold growth within the system and associated duct work. Excessive humidity can strain the operation of the unit’s design capabilities unless the system is configured with supplemental equipment to dehumidify the incoming outdoor air. Ambient air quality must also be considered, including areas where outdoor air pollution is high.
The use of ultraviolet germicidal irradiation (UVGI) may have application under specific use conditions, but the limitations must be considered. UVGI has been used to inactivate deposited viruses and bacteria in ducts and the upper air of rooms (e.g., hospitals). While these technologies may have application in certain healthcare settings, immediate implementation to aid with the current situation may be limited due to required modifications and existing system limitations.
The engineering controls discussed in this article are steps that can be immediately implemented in existing offices, schools, and other non-healthcare settings by occupational health professionals, building owners/operators, and employers to prevent SARS-CoV-2 transmission. While public health agencies continue to debate the merits of whether SARS-CoV-2 meets the criteria for airborne transmission, the mounting evidence supports the need for controlling aerosols in the indoor environment.
Specific guidance on engineering controls to reduce the risk of COVID-19 in non-healthcare buildings will be available from the American Industrial Hygiene Association (AIHA). Additional guidance is contained in a series of “back to work safely” documents developed for small businesses across 24+ industry sectors. To access these documents, visit www.backtoworksafely.org.
References
[1] CDC: “How COVID-19 Spreads.” https://www.cdc.gov/coronavirus/2019-ncov/prevent-getting-sick/how-covid-spreads.html
[2]UPI.com: “WHO reviewing evidence that indicates COVID-19 is airborne.” https://www.upi.com/Top_News/World-News/2020/07/08/WHO-reviewing-evidence-that-indicates-COVID-19-is-airborne/2781594188312/ Accessed July 9, 2020.
[3] WHO: Transmission of SARS-CoV-2: implications for infection prevention precautions (July 9, 2020).
[4] CDC: “Guidelines for Preventing the Transmission of Mycobacterium tuberculosis in Health-Care Settings, 2005.”
[5] CDC: Guidelines for Environmental Infection Control in Health-Care Facilities (2003; Updated July 2019). https://www.cdc.gov/infectioncontrol/pdf/guidelines/environmental-guidelines-P.pdf
[6] National Air Filtration Association: Understanding MERV | NAFA User’s Guide to ANSI/ASHRAE 52.2. https://www.nafahq.org/understanding-merv-nafa-users-guide-to-ansi-ashrae-52-2/
[7] ASHRAE: ASHRAE Position Document on Infectious Aerosols. (April 14, 2020).

Alex LeBeau, PhD, MPH, CIH, currently serves as the Secretary for the AIHA Indoor Environmental Quality committee. He is also the owner of Exposure Assessment Consulting, LLC, in Orlando, Florida, where he offers toxicology, industrial hygiene, risk assessment, and public health consulting services. Over his 13-year career, he has evaluated environmental and occupational exposures and has performed toxicological evaluations of chemicals and biological agents. In order to evaluate the exposure impacts on building occupants, he has performed indoor environmental quality assessments, including Legionella and water quality assessments, at healthcare, residential, and industrial facilities. He has served as a subject matter expert and has provided consultation on several occupational and environmental exposure claims. Finally, Dr. LeBeau has authored safety assessments on consumer products, including antimicrobial pesticide registration dossiers and Generally Recognized as Safe (GRAS) determinations for food ingredients following U.S. Food and Drug Administration (FDA) regulations for scientific procedures.