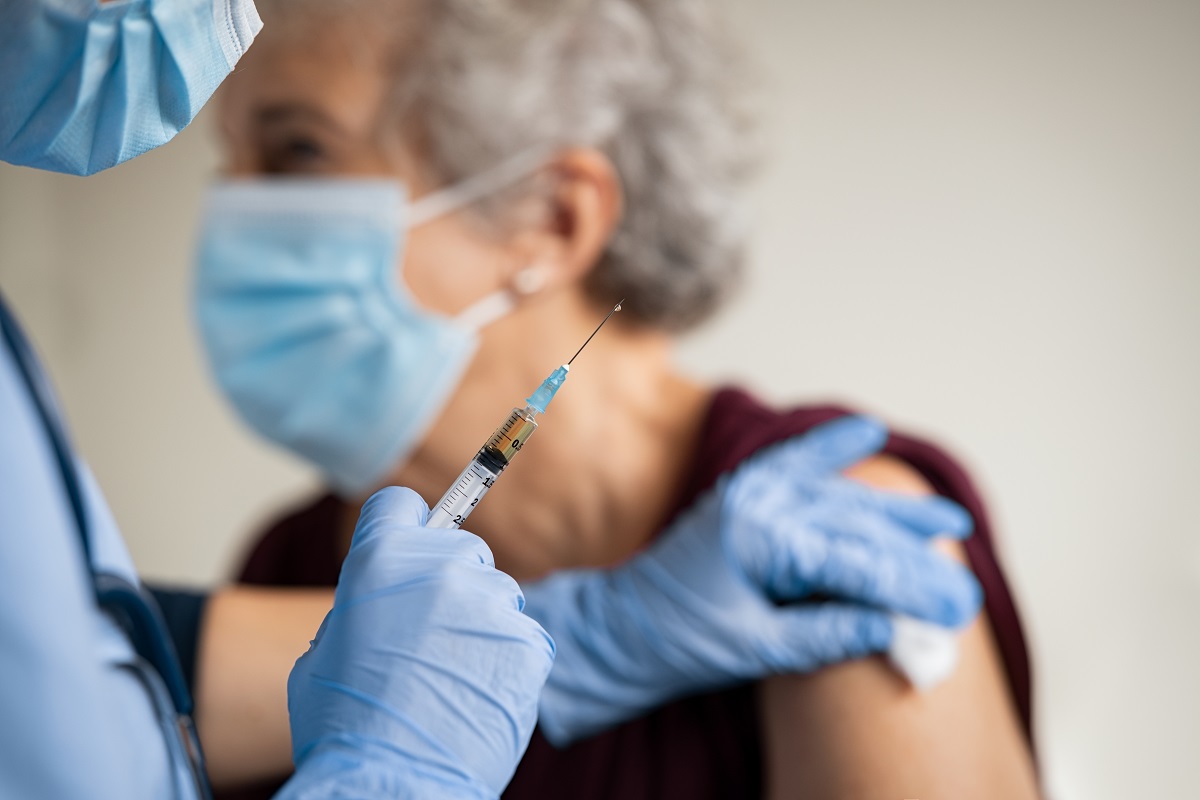
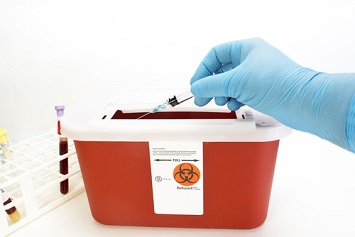
The ramping up of vaccination for COVID-19 comes with rising concerns about bloodborne pathogen (BBP) exposure for vaccinators and the need for personal protective equipment (PPE), as well as steps to prevent needlestick injuries and other BBP program requirements.
The Centers for Disease Control and Prevention (CDC) recommends that staff members at vaccination sites be provided with eye protection like goggles or face shields, gloves, and procedure face masks, but the federal PPE requirements for BBP exposures also include protective clothing like fluid-resistant gowns. The CDC does not recommend the use of N95 filtering facepiece respirators at vaccination sites.
The first several months of the COVID-19 pandemic brought a renewed focus on the need to prevent or mitigate workplace infectious disease hazards. While there is no federal Occupational Safety and Health Administration (OSHA) standard for infectious diseases, the Obama administration OSHA initiated an infectious disease rulemaking it never completed. California has had an airborne transmissible disease (ATD) standard that only applies to correctional facilities and jails, healthcare and long-term care facilities, and public services like emergency medical service, fire, and law enforcement agencies.
Virginia now has a permanent infectious disease standard for COVID-19. Oregon has an emergency temporary standard (ETS) and recently proposed a permanent standard that may take effect when its ETS expires. California and Michigan both have temporary COVID-19 standards.
However, now that COVID-19 vaccines are available under a Food and Drug Administration (FDA) emergency use authorization, there is a renewed focus on BBP exposures and needlestick injury prevention. November 2020 marked the 20th anniversary of the passage of the federal Needlestick Safety and Prevention Act (PL 106-430) into law.
Needlestick Injuries, BBP Regulations
An estimated 600,000 to 800,000 needlestick injures occur each year, according to the National Institute for Occupational Safety and Health (NIOSH).
OSHA’s BBP standard (29 CFR 1910.1030) covers exposures to pathogens that include hepatitis B virus (HBV), hepatitis C virus (HCV), and human immunodeficiency virus (HIV), the virus that causes acquired immunodeficiency syndrome (AIDS), found in blood and other bodily fluids. The BBP standard includes requirements to establish and implement an exposure control plan that must be updated annually; use universal protections; identify and use both engineering and work practice controls; provide PPE that includes gloves, fluid-resistant gowns, and eye protection; provide hepatitis B vaccination for exposed employees; provide information and training about BBPs and sharps safety; provide postexposure evaluation and follow-up care; and maintain a sharps injury log.
Employers must clean, repair, and replace PPE as necessary. PPE must be provided, maintained, repaired, and replaced at no cost to the worker.
The sharps injury logs required under OSHA’s BBP standard must at least include information about the date of the injury, the case report number, the type of device involved, the device brand name, the department or work area where the injury occurred, and a brief description of how the sharps injury occurred. However, a log also may include additional information like the occupation of the worker injured and the type of sharps injury protection feature on the device.
BBP engineering controls include sharps disposal containers, self-sheathing needles, and safer medical devices, such as sharps with engineered sharps-injury protection. Work practices in BBP programs include reducing the possibility of exposure by changing the way a task like vaccine administration is performed, implementing appropriate practices for handling and disposing of contaminated sharps, handling site laundry, and cleaning contaminated surfaces and items.
The National Institute of Environmental Health Sciences (NIEHS) cautions employers engaged in COVID-19 vaccination programs to ensure the safety of vaccinators and vaccine administrators in preventing needlesticks and blood exposures. The NIEHS recommends the implementation of engineering and administrative controls, including PPE such as face coverings, eye protection, and gloves.
The FDA regulates surgical or “procedure” masks used as an infection source control and respirators used in healthcare facilities under its medical device authority. NIOSH evaluates and approves all respirators but not surgical masks or nonregulatory face coverings.
Needlestick injuries most often occur, according to the NIEHS, in hospital settings when disposable syringes are used but also may occur in nursing homes and other workplace settings. There is little research into or knowledge of needlesticks during mass vaccination programs.
Inexperienced vaccinators can have the highest rate of needlestick injuries. Factors involved in needlestick injuries often include:
- Distraction caused by noise and crowded spaces.
- Difficulty removing the cap of a needle or difficulty in recapping.
- Lack of an ideal vaccination site—mass vaccination sites have been set up by state and local health departments in arenas, parking lots, and stadiums.
- The patient receiving a vaccine jumps, jars, or moves.
- Inappropriate technique, such as pinching skin with opposite hand.
- Inappropriate needle size or type.
- Lack of an engineered sharps injury prevention feature on a syringe or needle.
- Improper disposal of syringes or placing a used syringe on a surface.
- Vaccinator fatigue after immunizing patients over several consecutive workdays.
Requirements of OSHA BBP Standard
OSHA recommends that all employers at COVID-19 vaccination sites with recognizable BBP hazards take steps to prevent needlesticks and BBP exposure, including PPE for protection from the SARS-CoV-2 virus that causes COVID-19, human blood, and other potentially infectious material. The agency also recommends the use of sharps with engineered sharps injury protections or other safer needle devices such as retractable and self-sheathing needles. OSHA also recommends that vaccination site workers not recap, shear, or break contaminated needles or pass used sharps between workers.
The agency recommends the use of sharps containers located nearby that are closable, puncture-resistant, leakproof, and biohazard-labeled and/or color-coded to allow for immediate disposal of used syringes and other sharps.
OSHA also encourages workers at vaccination sites to report safety and health concerns, including concerns about needlestick injuries, to their immediate supervisors.
Employers will need to assess the risk of blood exposures and needlesticks for frontline vaccinators at each vaccination location and document the exposures in their exposure control plans. They will need to perform a hazard assessment before a vaccination program is launched to determine exposure, evaluating both the work environment and work practices utilized at the vaccination sites.
All vaccinators should be trained in the proper activation of the sharps-injury prevention features of syringes or needles before use. Employers must ensure that puncture-resistant sharps containers are located as close as possible to the point of sharps use and that all used syringe and needle devices can be properly disposed of. The sharps container should be labeled, color-coded, secured to prevent tipping over, and closed and replaced when three-fourths full.
BBP planning for vaccination sites also must include site-specific procedures for accessing medical follow-up for vaccinators who experience a needlestick injury. Employers should arrange for postexposure care as soon as possible after a needlestick injury, ideally immediately following the incident. The CDC recommends that postexposure prophylaxis (PEP) for exposure to human immunodeficiency virus (HIV) should begin as soon as possible and no later than 72 hours after the incident.
PEP
The costs involved in needlestick injuries, follow-up monitoring, and PEP for BBPs can be significant. Required follow-up includes medical assessment of the injury, source patient testing, counseling, administration of PEP (if necessary), and continued postexposure follow-up by the medical provider at specific intervals. The CDC and U.S. Public Health Service have employer guidance for testing and clinical management of occupational exposures to HBV, HCV, and HIV.
The likelihood of BBP exposures can change as the groups eligible for vaccination expand. Researchers estimate that members of the Baby Boomer generation may be five times more likely to have hepatitis C infections.
Organizations sponsoring vaccination should clearly state that any contractors or volunteers mobilized as vaccinators are included in their postexposure BBP protocols, including follow-up medical care. Needlestick injuries are considered work-related and typically are covered by state workers’ compensation laws.
The CDC has a workbook for designing, implementing, and evaluating sharps injury prevention programs in healthcare settings. Steps in the CDC’s workbook include developing the organizational capacity for a sharps injury prevention program, assessing program operations, preparing a baseline profile of sharps injury and prevention activities, determining intervention priorities, developing and implementing action programs, and monitoring program performance.
NIOSH ‘Stop Sticks’
Other resources include NIOSH’s “Stop Sticks” Campaign that offers information about the knowledge, behaviors, and attitudes of workers and the use of devices with sharps injury protection. The Stop Sticks campaign is updated and maintained by NIOSH’s National Occupational Research Agenda Healthcare and Social Assistance Sector Council and includes newsletters, posters, and videos for use in the workplace.
Needlestick injuries have frequently occurred due to attempts to recap needles and failing to dispose of used needles into a puncture-resistant sharps container, according to NIOSH. The institute recommends that those who work with syringes and needles:
- Get a hepatitis B vaccination and participate in any training provided in infection prevention.
- Use devices with safety features provided by the employer.
- Plan for safe handling and disposal of needles before using them.
- Avoid recapping needles.
- Promptly dispose of used needles in an appropriate sharps disposal container.
- Report all needlestick and sharps-related injuries promptly to ensure that you receive appropriate follow-up care.
- Report any needlestick hazards you observe to your employer.
There are no vaccines available to prevent hepatitis C or HIV infection.
OSHA has a model exposure control plan that can be used as a template for BBP compliance. The agency’s Enforcement Procedures for the Occupational Exposure to Bloodborne Pathogens also are available on OSHA’s website. Enforcement procedures include instructions for compliance safety and health officers (CSHOs) at multiemployer worksites. A CSHO will review the exposure control plan and sharps injury log during an inspection.
If you have employees with recognized exposures to blood and other potentially infectious materials like bodily fluids, you need an exposure control program and must update it annually. You also need to maintain a sharps injury log in addition to your OSHA 300 log of occupational injuries and illnesses. You will need to supply them with PPE, including eye protection like goggles or face shields, gloves, and fluid-resistant gowns. You will need to provide sharps with engineered sharps injury protections and sharps disposal containers, as well as information and training about sharps injury prevention.